Today more and more manufacturers of medical device parts are using PEEK (polyetheretherketone). Why PEEK? Because this material is bio-compatible, inert to body fluids, and can be processed relatively easy with high precision micro molding for individual implants or surgical instruments.
For medical device parts, PEEK provides a great benefit: It saves weight, allows more design freedom and a greater functional integration, it also scores with X-ray transparency and elasticity which corresponds approximately to bone. At the same time it is a lower cost alternative to Titan the classic implant material, especially when using medical instruments or endoscopes, where the good electrical insulation properties of PEEK come into play.
Medical device parts that are intended for long-term contact with body tissues must meet particularly high quality requirements for registration in Europe or in the US. The manufacturers have to demonstrate on the one hand, that the raw materials are suitable for the respective area of application, and on the other hand demonstrate how they ensure consistent quality. For example, in further processing using injection molding, different cooling speeds influence the material properties of PEEK. Also, the duration and temperature of the heat treatment has a direct influence on the crystal properties of the PEEK polymers and thus their mechanical properties. That is on the one hand, that the material properties can be specifically controlled, on the other hand also, that any variation in the production process changes the quality of the parts. So it is evidently important to stabilizing every relevant process conditions to get over a long production time of only high quality parts.
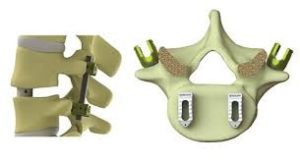
The complete detachment of ligaments, tendons or other soft tissues from their associated bones within the body is relatively commonplace injuries, particularly among athletes. Such injuries are generally the result of excessive stresses being placed on these tissues. For example, tissue detachment may occur as the result of an accident such as a fall, over-exertion during a work-related activity, during the course of an athletic event, or activities. For a complete detachment, however, surgery may be needed to re-attach the soft tissue to its associated bone or bones. Numerous devices made out of PEEK material are currently available to re-attach soft tissue to bone. Examples of such currently-available medical device parts include screws, staples, suture anchors and tacks
By using the latest micro molding technology gives a significant improvement of part quality and confirmed this with simultaneous cost reduction due to faster cycle time and smaller sprue. The improved long-term stability of the production process allowed additionally reducing operator time. It is relatively straightforward to process PEEK as long as you know exactly your process window and equipment. Strict operating procedures are recommended for heating up and shutting down the machine cell enabling to reach a proper and stable productions process.
Let us know how we can help design and manufacture your next medical device or parts to be made from PEEK contact us
Re-posted from http://www.medicalplasticsnews.com/news/close-to-the-bone-why-manufacturers-are-using-peek-products/ from 12/27/16 posting